The ASEAN Agreement on Medical Device Directive (AMDD) is a crucial step towards harmonizing medical device regulations across Southeast Asia. This agreement aims to facilitate trade, enhance patient safety, and promote innovation in the medical device sector within the ASEAN region. This comprehensive guide delves into the key aspects of the AMDD, its implications, and its significance for the future of healthcare in Southeast Asia.
What is the AMDD?
The AMDD establishes a common set of rules and regulations for medical devices across all ten ASEAN member states. This harmonization effort aims to simplify the registration process for medical devices, ensuring consistent standards of safety and quality throughout the region. The agreement covers a broad spectrum of medical devices, from simple bandages to complex diagnostic equipment, emphasizing essential principles like pre-market notification, post-market surveillance, and adverse event reporting. By creating a more predictable and transparent regulatory landscape, the AMDD encourages investment and innovation in the medical device industry within ASEAN.
Key Features of the ASEAN Medical Device Directive
The AMDD focuses on several key areas to streamline the medical device regulatory process:
- Pre-Market Notification: Manufacturers must notify the relevant authorities in each member state before introducing a new medical device to the market. This process ensures that the device meets the established safety and performance requirements.
- Post-Market Surveillance: Even after a device is approved, manufacturers are required to monitor its performance and report any adverse events or safety concerns that arise. This ongoing surveillance helps identify potential risks and protect patient safety.
- Essential Principles: The AMDD outlines a set of essential principles that all medical devices must adhere to, focusing on safety, efficacy, and quality. These principles serve as the foundation for the entire regulatory framework.
- Conformity Assessment: The agreement establishes procedures for conformity assessment, ensuring that medical devices comply with the essential principles and technical requirements.
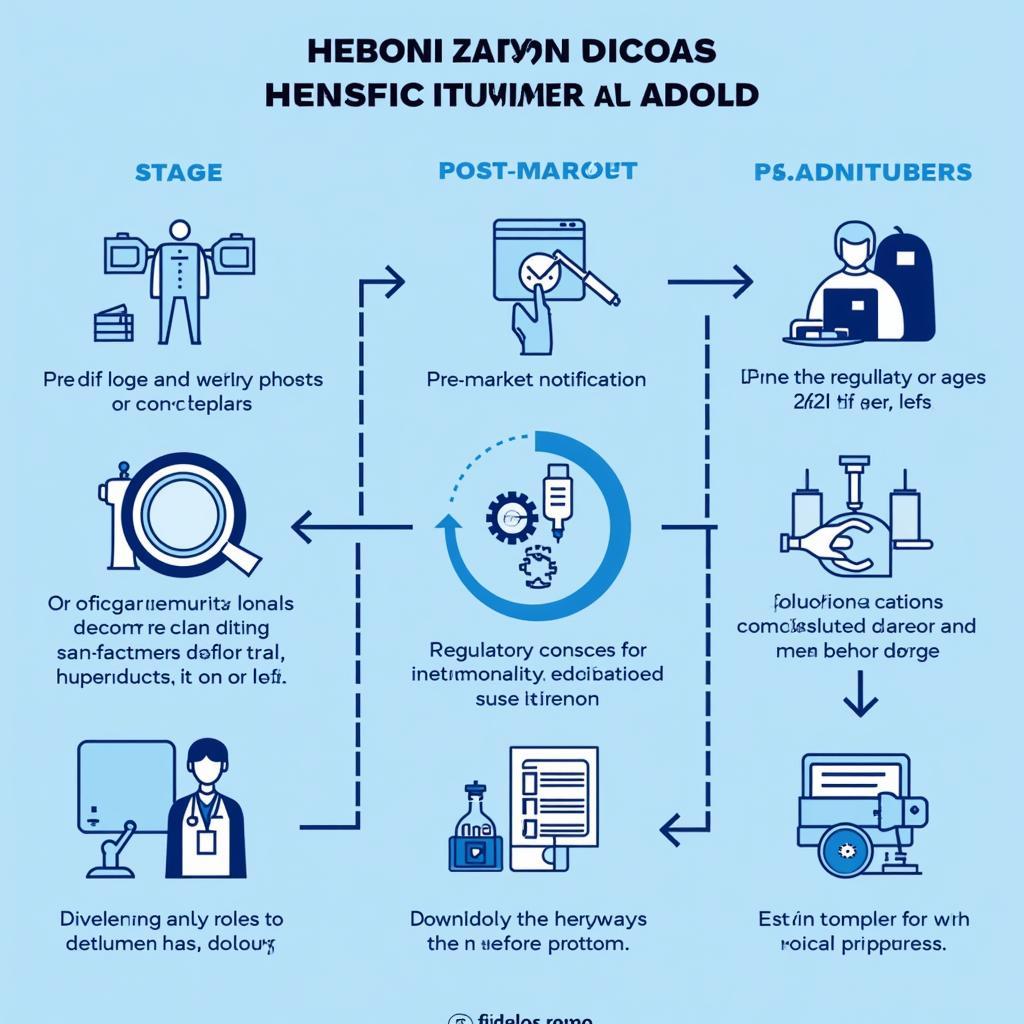 ASEAN Medical Device Directive Harmonization Process
ASEAN Medical Device Directive Harmonization Process
Benefits of the ASEAN Medical Device Directive
The AMDD brings numerous benefits to patients, manufacturers, and the ASEAN region as a whole:
- Enhanced Patient Safety: By establishing common standards and procedures, the AMDD ensures that medical devices used within ASEAN meet consistent safety and quality requirements, protecting patients from potential harm.
- Facilitated Trade: The harmonized regulatory framework simplifies the process for manufacturers to register and market their devices across multiple ASEAN countries, promoting regional trade and economic growth.
- Increased Investment: The predictable and transparent regulatory environment fostered by the AMDD encourages investment in the medical device industry within ASEAN, leading to innovation and improved healthcare access.
- Strengthened Regional Cooperation: The AMDD represents a significant achievement in regional cooperation, demonstrating the commitment of ASEAN member states to work together to improve healthcare outcomes.
Challenges and Opportunities
While the AMDD offers significant advantages, several challenges need to be addressed:
- Implementation Variations: Differences in interpretation and implementation of the AMDD across member states could create inconsistencies and hinder the full realization of its benefits.
- Capacity Building: Strengthening regulatory capacity in some member states is crucial to ensure effective implementation and enforcement of the AMDD.
- Public Awareness: Raising public awareness about the AMDD and its implications for patient safety is essential to build trust and confidence in the regulatory system.
“The AMDD represents a pivotal moment for healthcare in ASEAN,” says Dr. Anya Sharma, a leading expert in medical device regulation. “By creating a harmonized regulatory framework, the agreement paves the way for safer, more accessible, and innovative healthcare solutions across the region.”
Looking Forward
The AMDD holds immense potential for transforming the medical device landscape in ASEAN. Continued collaboration among member states, coupled with effective implementation and ongoing refinement of the regulatory framework, will be critical to maximizing its positive impact on healthcare in the region. “The AMDD’s success hinges on continuous dialogue and collaboration between regulators, manufacturers, and healthcare professionals,” adds Dr. Sharma. “This collective effort will ensure that the agreement truly delivers on its promise of enhanced patient safety and a thriving medical device sector.”
In conclusion, the ASEAN Agreement on Medical Device Directive (AMDD) signifies a major step towards a more integrated and efficient medical device market in Southeast Asia. By harmonizing regulations, promoting safety, and facilitating trade, the AMDD promises to improve healthcare outcomes and stimulate innovation in the region.
FAQs
- What is the purpose of the AMDD? To harmonize medical device regulations across ASEAN.
- Who benefits from the AMDD? Patients, manufacturers, and the ASEAN economy.
- What are the key features of the AMDD? Pre-market notification, post-market surveillance, essential principles, and conformity assessment.
- What are the challenges in implementing the AMDD? Variations in implementation, capacity building, and public awareness.
- How can I learn more about the AMDD? Contact your local regulatory authority or visit the ASEAN Secretariat website.
- What does AMDD stand for? ASEAN Agreement on Medical Device Directive
- Which countries are involved in the AMDD? All ten ASEAN member states.
Need support? Contact us 24/7: Phone: 0369020373, Email: aseanmediadirectory@gmail.com, or visit us at: Thon Ngoc Lien, Hiep Hoa, Bac Giang, Vietnam.
You might also be interested in these related articles:
- ASEAN’s Growing Healthcare Market
- Medical Device Registration in ASEAN: A Step-by-Step Guide
- Investing in ASEAN’s Medical Device Industry
If you have further questions, please don’t hesitate to reach out.
